Ophthalmic Viscosurgical Devices (OVDs) are essential tools in modern cataract and other intraocular surgeries. They provide critical functions such as maintaining space, protecting ocular tissues, and facilitating the manipulation of intraocular structures. Alsanza, a prominent manufacturer in the field, offers a range of OVDs under the brand name Alsavisc, with concentrations tailored to specific surgical needs.
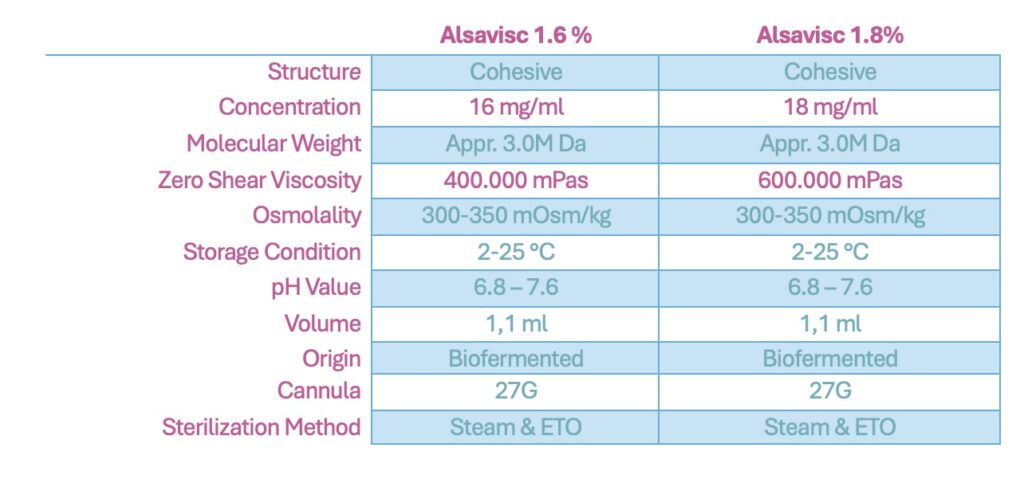
Alsavisc 1.6% and 1.8% are cohesive OVDs containing sodium hyaluronate at 16 mg/ml and 18 mg/ml, respectively. These products are derived from non-animal sources through recombinant DNA (rDNA) bio-fermentation methods. This advanced biotechnology process utilizes transgenic microorganisms that express human hyaluronic acid (HA) genes, resulting in pure hyaluronate with a molecular weight of approximately 3 million Daltons. The organisms secrete HA directly into an isotonic medium, minimizing the presence of allergens, endotoxins, and exotoxins and reducing the need for extensive purification processes.
Alsavisc 1.6% has a zero-shear viscosity (ZSV) of approximately 400,000 mPas, while Alsavisc 1.8% has a ZSV of around 600,000 mPas. These viscosity levels are designed to provide optimal space maintenance and stability within the anterior chamber during surgical procedures. The cohesive nature of these OVDs allows for easy removal at the end of surgery, reducing the risk of postoperative complications associated with retained viscoelastic material.
The high molecular weight and purity of the hyaluronic acid in Alsavisc OVDs contribute to their excellent coating and retention properties. These provide effective protection for corneal endothelial cells during phacoemulsification. Additionally, the homogeneous character of the long HA chains ensures consistent performance, enhancing the safety and efficacy of the surgical procedure.
In summary, Alsavisc 1.6% and 1.8% OVDs from Alsanza offer surgeons reliable and effective options for maintaining anterior chamber stability, protecting ocular tissues, and facilitating intraocular manipulations during cataract and other intraocular surgeries. Their non-animal, bio-fermented origin ensures high purity and biocompatibility, aligning with modern standards for surgical materials.
If you have any questions about Alsavisc or would like to arrange a trial, please contact Medical DevEyes